A biosimilar is a biologic that is highly similar to and has no clinically meaningful differences in terms of safety, purity, and potency from an approved biologic (the biosimilar’s “reference product”). Similarity is established between the biosimilar and the reference product based on the totality of evidence.1, 2
References:
- U.S. Food and Drug Administration. Biological Product Definitions. 2019. Available at: https://www.fda.gov/media/108557/download. Accessed November 14, 2019.
- U.S. Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. April 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed November 14, 2019.
Fast Facts About
Biological Drugs
Fast Facts About
Biological Drugs
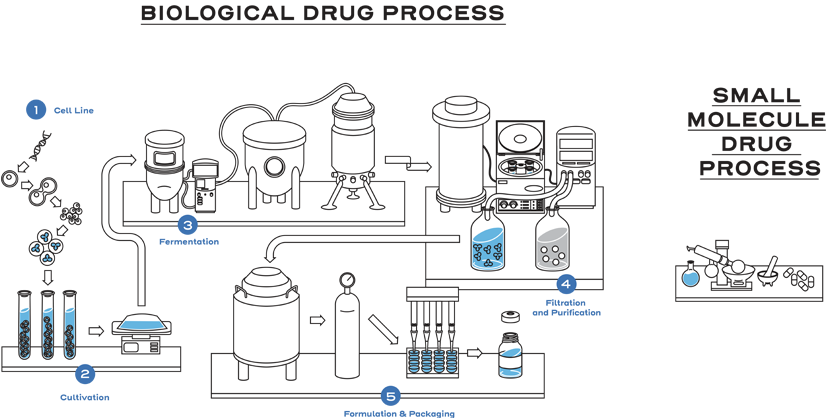
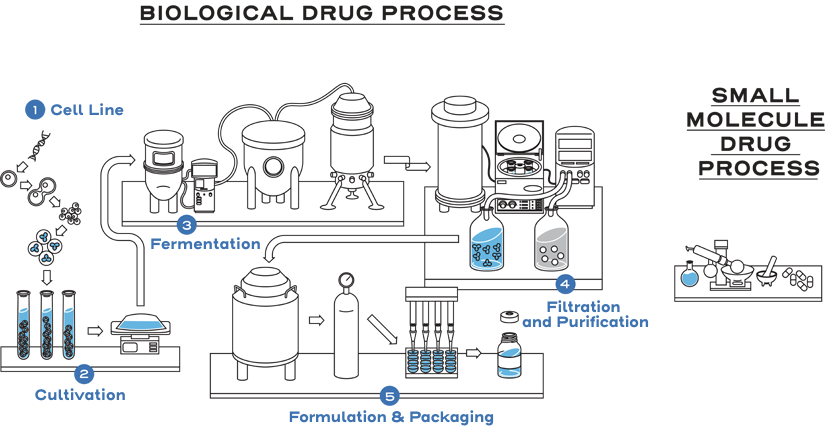
Biological drugs are large, complex proteins that are generally manufactured in living cells.1
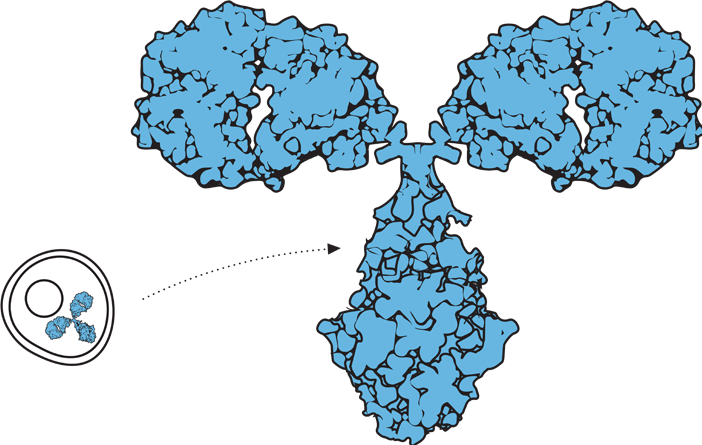
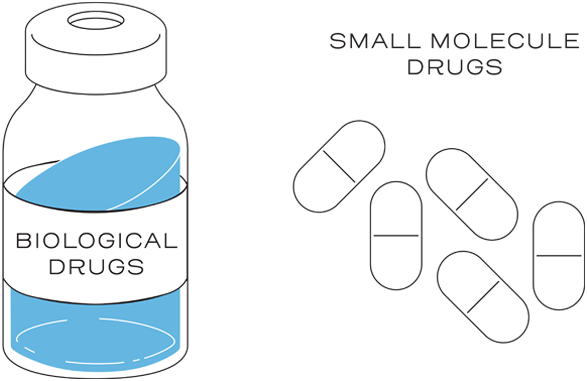
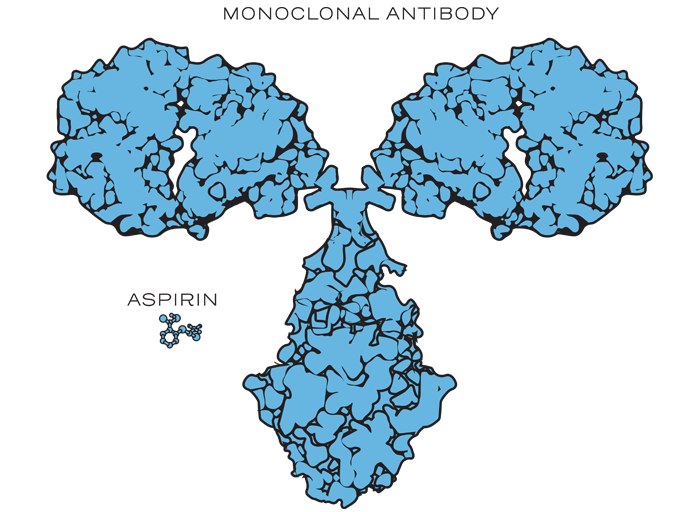
Biological drugs, such as monoclonal antibodies, are up to 1,000 times the size of a small-molecule drug, such as aspirin.1,2
The nature of biological drugs and their manufacturing process leads to unique attributes in the product being produced.1
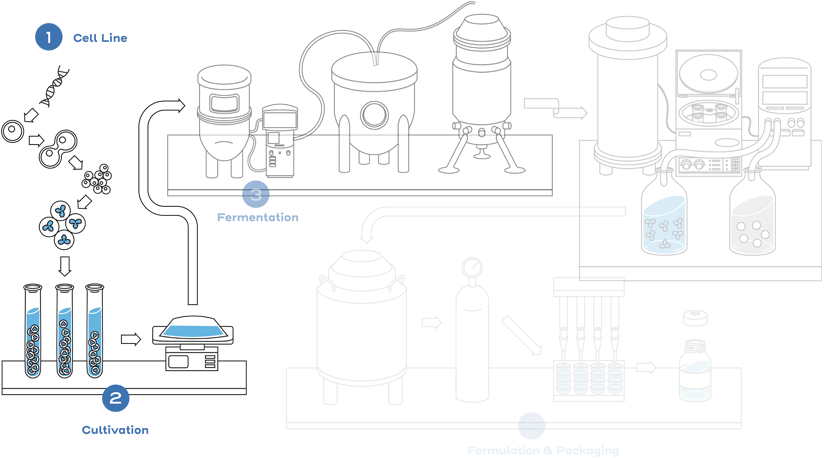
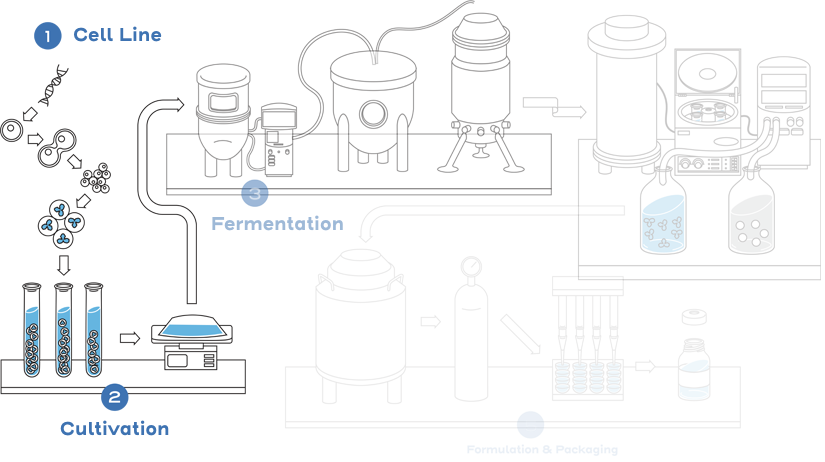
Given the inherent variability associated with biological product manufacturing and the fact that the manufacturing processes for a reference product and a biosimilar will be different, a biosimilar will be highly similar to its reference product, but not identical. Small differences in structure are expected and acceptable, provided that these differences do not lead to clinically meaningful differences that impact safety or efficacy.1
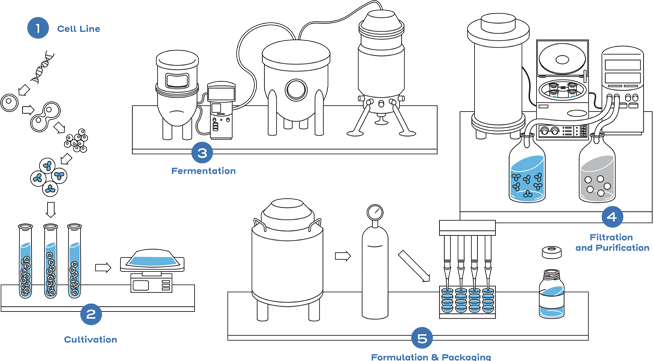
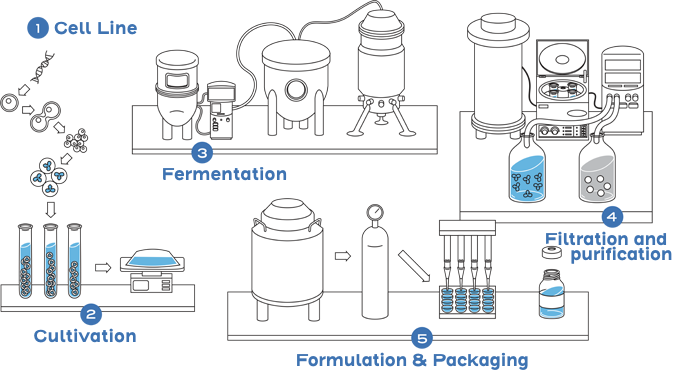
This is not the case with small-molecule drugs, which are typically developed through chemical synthesis. The chemical reactions are predictable and can be controlled to replicate an identical copy.1,2
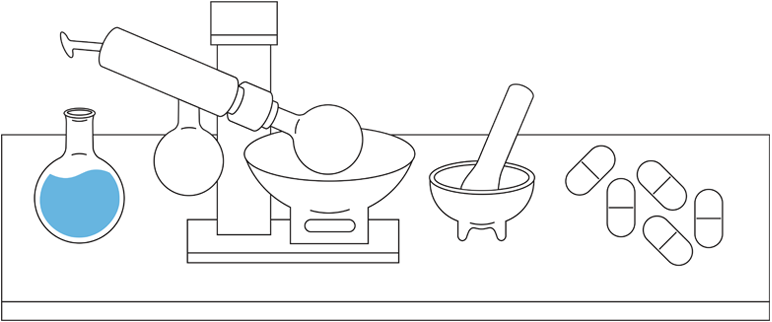
The size, complexity, stability, and inherent variability within the manufacturing process are why biological products are different from small-molecule drugs.1
References:
- U.S. Food and Drug Administration. Biological Product Definitions. 2019. Available at: https://www.fda.gov/media/108557/download. Accessed November 14, 2019.
- Sekhon, BS, Saluja V. Biosimilars. 2011:1-11
Fast Facts About
Biological Drugs
Fast Facts About
Biological Drugs
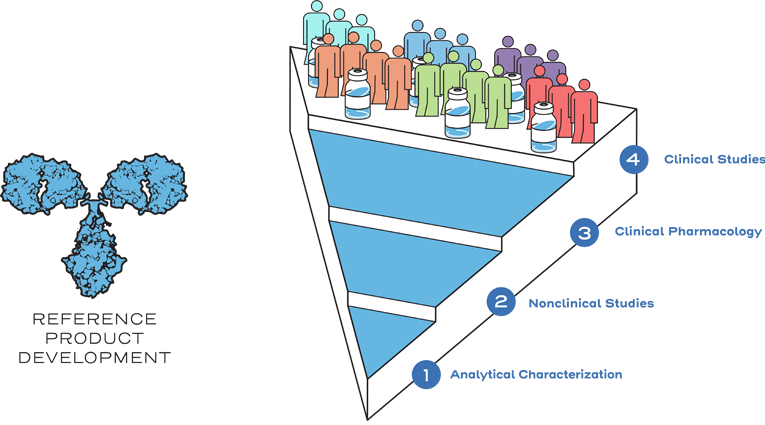
The goal of biosimilar development is to create a biological drug that is highly similar to a previously approved reference product...1
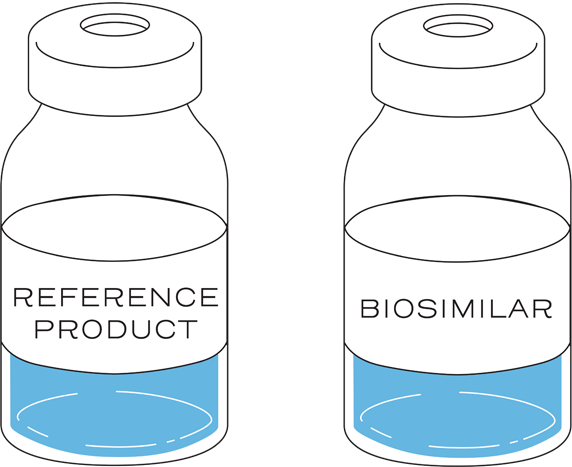
...with no clinically meaningful differences in safety, purity, and potency.1

The reference product manufacturing process is a proprietary, multi-step process.1, 2
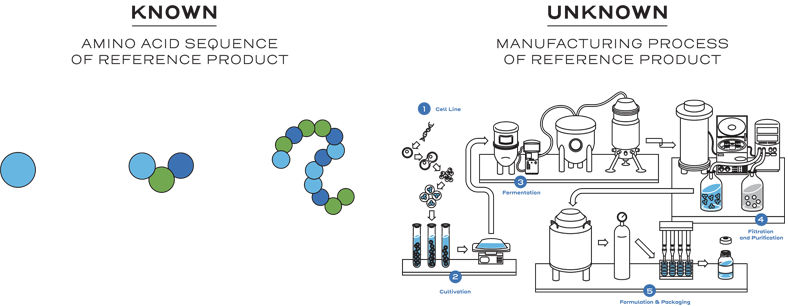
This means biosimilar manufacturers must develop an entirely new customized process.1
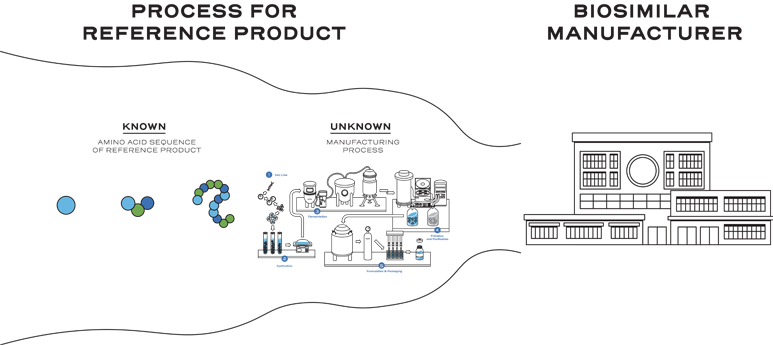
This means biosimilar manufacturers must develop an entirely new customized process.1
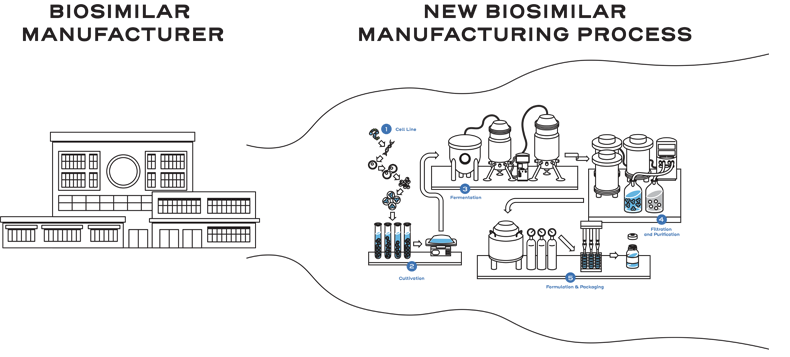
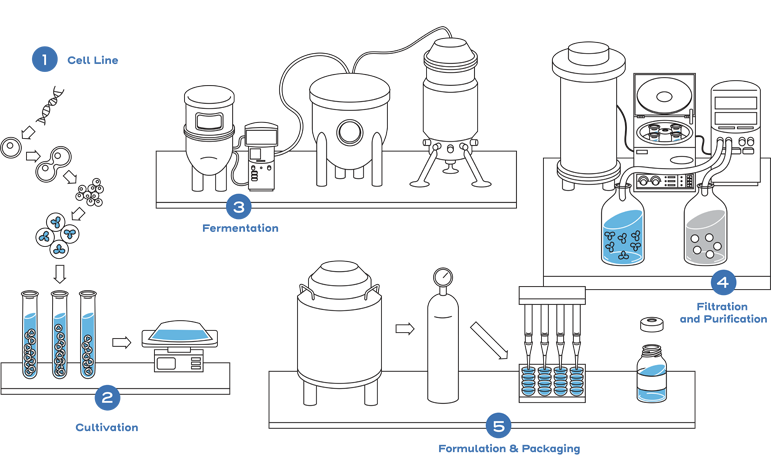
This process includes developing a unique cell line, establishing a master cell bank, cell culture, expansion, isolation, purification, characterization, stability, formulation, fill, and finish.2
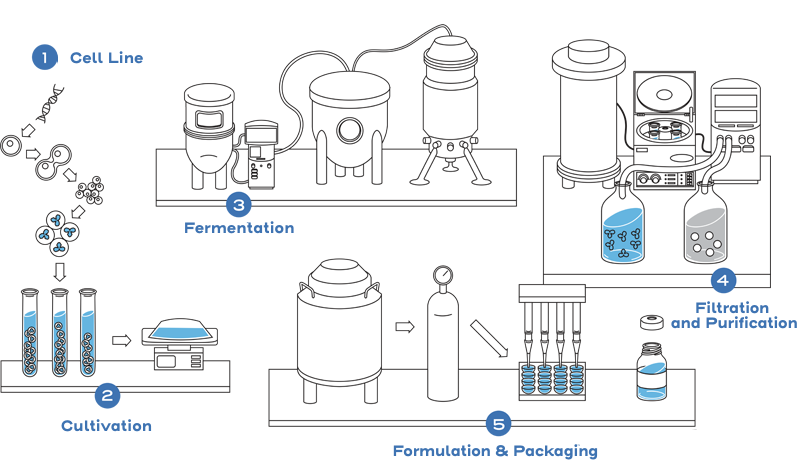
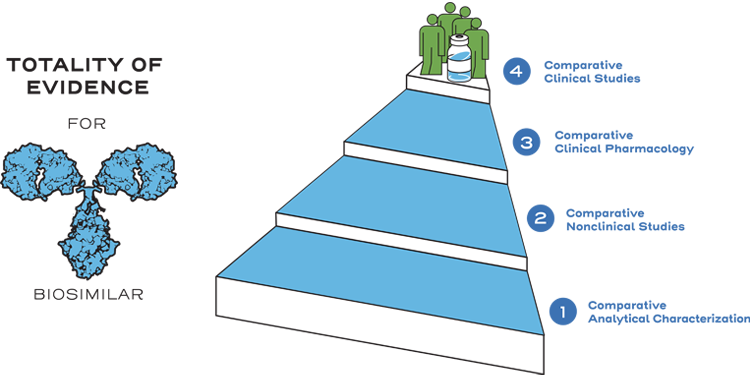
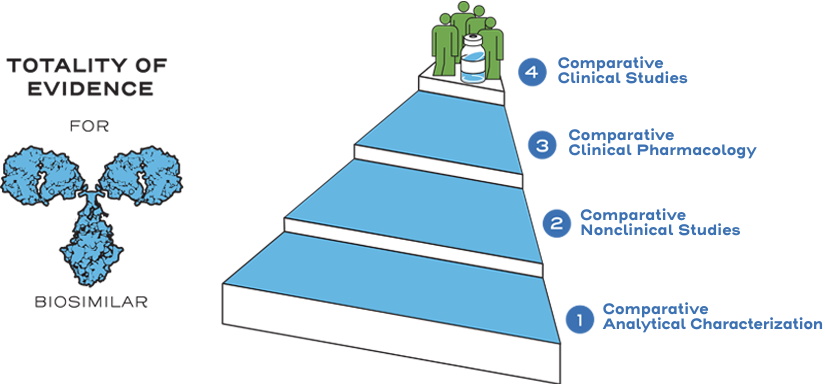
Instead of generating the same full profile of nonclinical and clinical data as the reference product, biosimilarity is established between the biosimilar and the reference product based on the totality of evidence. The manufacturer may rely on the national regulatory agency’s previous findings of safety and efficacy for the reference product through the comparative data showing similarity between the products. Efficiencies are realized through an abbreviated approval pathway.1
References:
- U.S. Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. April 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed November 14, 2019.
- Sekhon, BS, Saluja V. Biosimilars. 2011:1-11
Fast Facts About Extrapolation
Fast Facts About Extrapolation
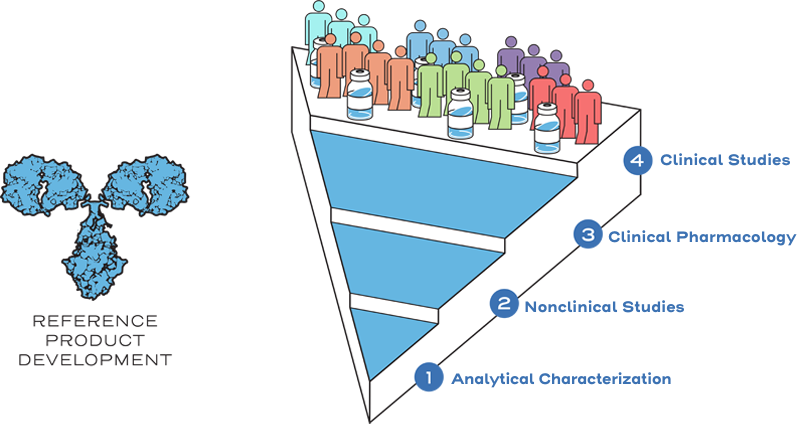
Reference products, also known as originator biologics, are approved on the basis of demonstrating safety, effectiveness, and substantial evidence for a new product.1
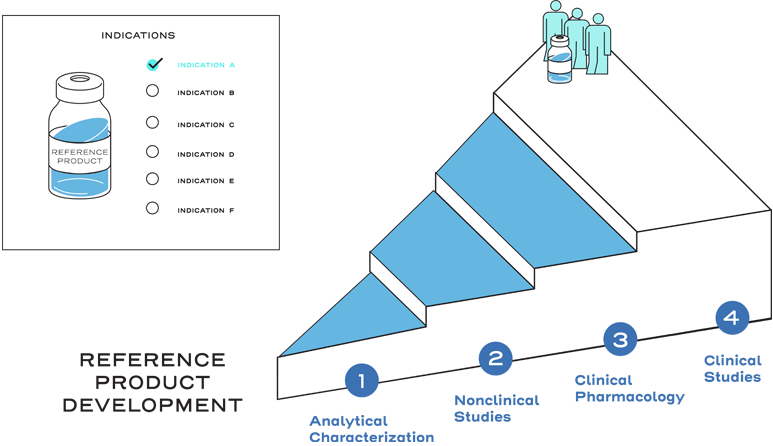
This approval process includes a full profile of nonclinical and clinical data, and originator biologics must demonstrate safety and effectiveness in clinical trials to gain approval for each indication sought.2
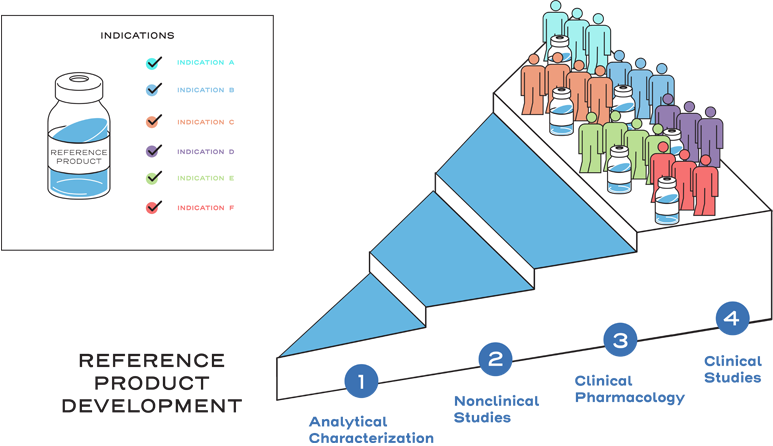
This approval process includes a full profile of nonclinical and clinical data, and originator biologics must demonstrate safety and effectiveness in clinical trials to gain approval for each indication sought.2
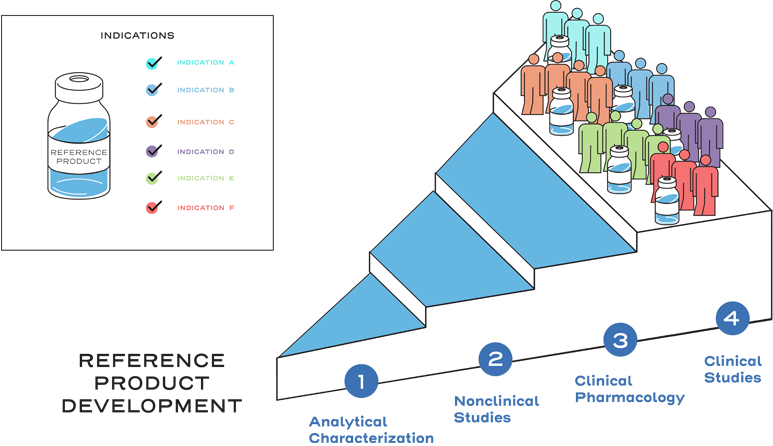
Similarity is established between the biosimilar and the reference product based on the totality of evidence, including comparative analytical (structural and functional) characterization, nonclinical evaluation, pharmacokinetic (exposure)/pharmacodynamic (response) data, and additional comparative clinical studies.3
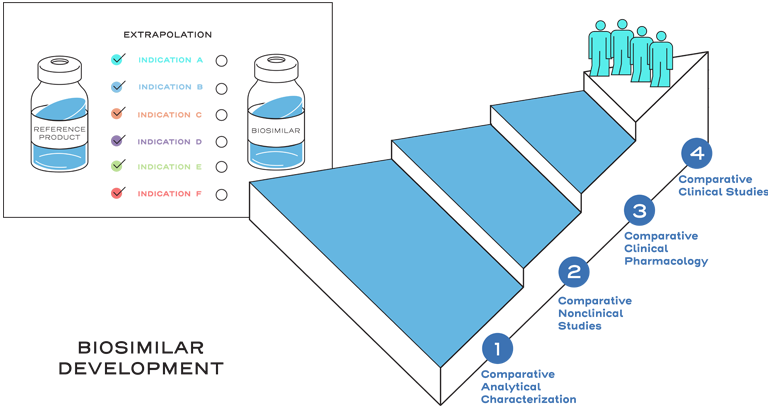
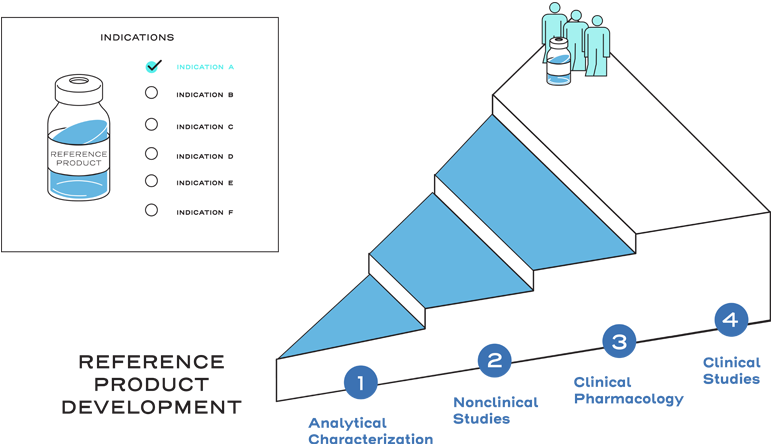
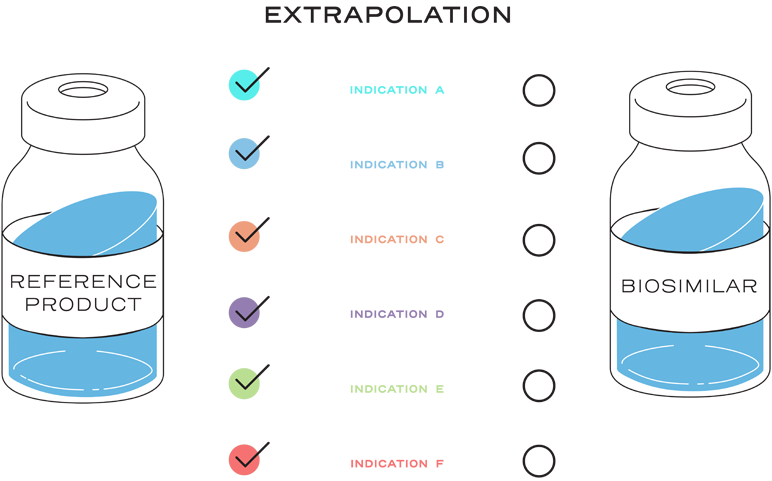
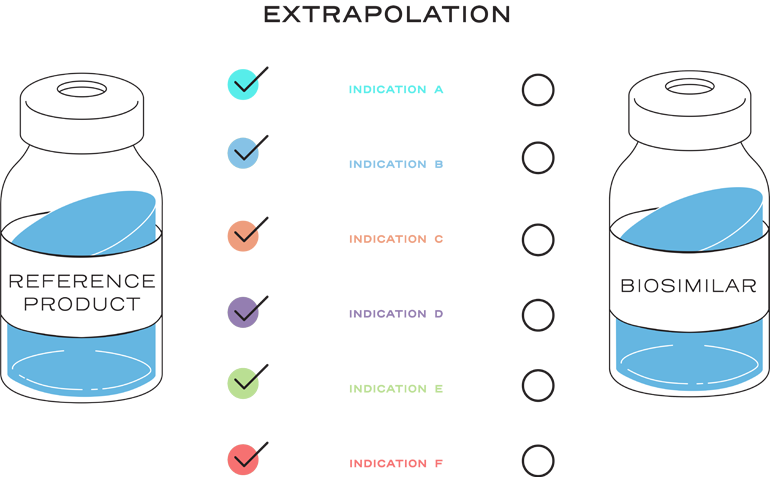
Extrapolation is the approval of a proposed biosimilar product in one or more additional indications for which the reference product is licensed, but for which the biosimilar has not been studied in clinical trials.2
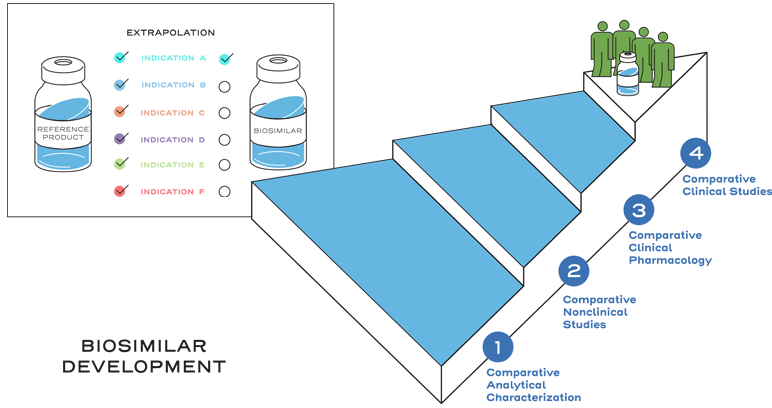
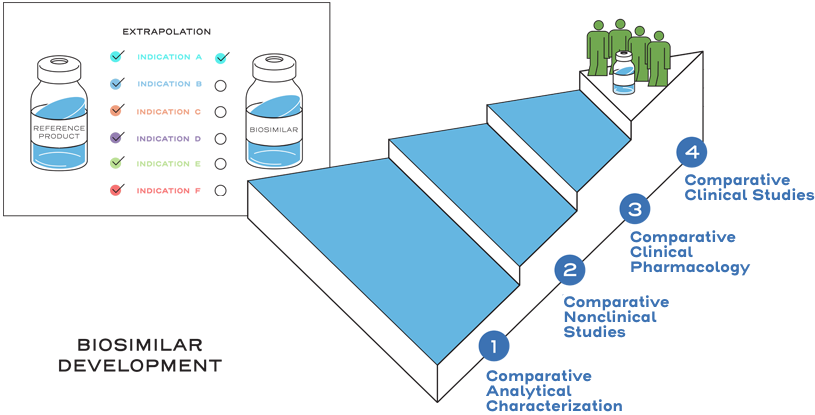
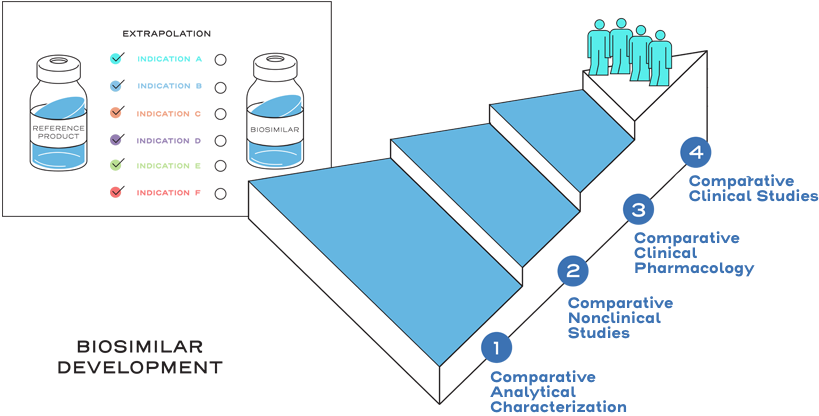
Extrapolation is not automatic.2
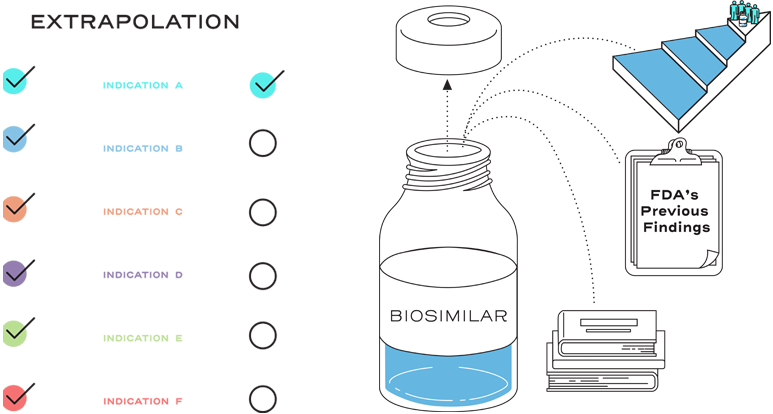
Extrapolation is based on all the available data in the biosimilar application and the FDA’s previous findings of safety and efficacy for the other approved indications of the reference product.2
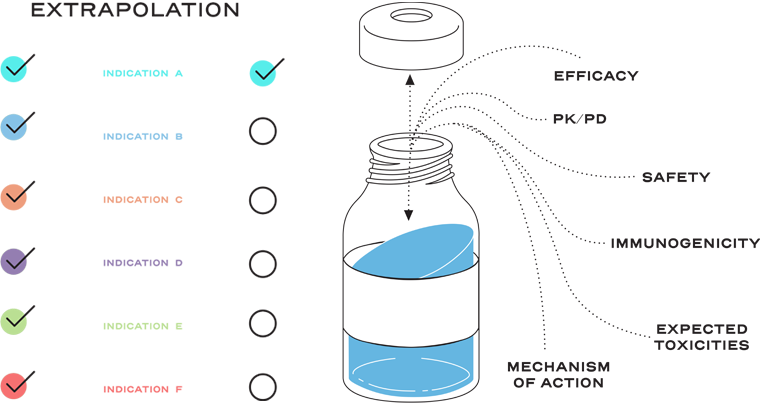
It is also based on knowledge and consideration of various scientific factors, such as mechanism of action, pharmacokinetics, pharmacodynamics, immunogenicity, expected toxicities, safety, and efficacy, for each indication sought.2
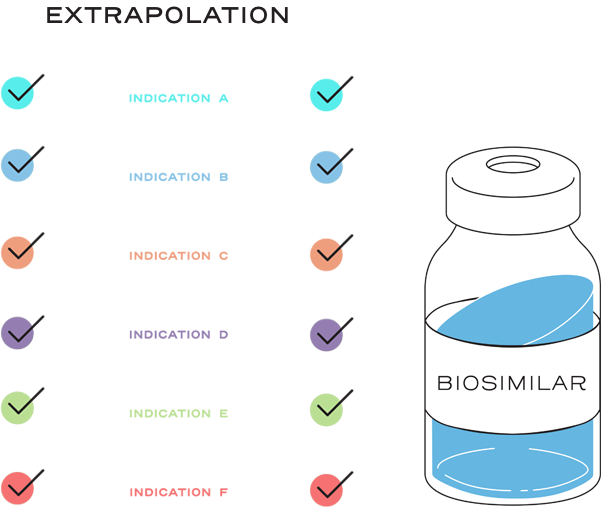
Extrapolation reduces or eliminates the requirement to study a proposed biosimilar with clinical trials in every indication of the reference product.2
References:
- U.S. Food and Drug Administration. Biological Product Definitions. 2019. Available at: https://www.fda.gov/media/108557/download. Accessed November 14, 2019.
- U.S. Food and Drug Administration. Biosimilar Product Regulatory Review and Approval. 2019. Available at: https://www.fda.gov/media/108621/download. Accessed November 14, 2019.
- U.S. Food and Drug Administration. Guidance for Industry: Scientific Considerations in Demonstrating Biosimilarity to a Reference Product. April 2015. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed November 14, 2019.